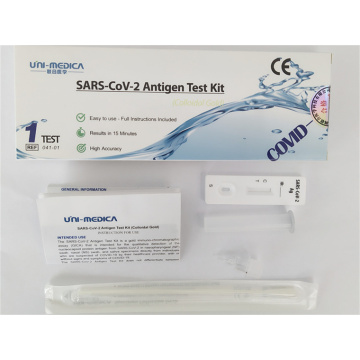
2019-nCoV Ag Rapid Test Card
| Min. Order: | 10000 Piece/Pieces |
|---|
| Packaging: | export standard |
|---|---|
| Productivity: | 100,000,000 pcs/year |
| Brand: | Uni-medica |
| Transportation: | Ocean,Land,Air,Express |
| Place of Origin: | China |
| Supply Ability: | 100,000,000 pcs/year |
| Certificate: | CE/SGS |
| HS Code: | 38220090 |
| Port: | Shenzhen,Guangzhou,Hongkong |
Basic Info
Model No.: R041-01
Click on the follow link to find out more information: https://www.unimed-global.com/antigen-test-kit-of-covid-19/
Company Info
- Company Name: Shenzhen Uni-medica Technology Co.,Ltd
- Representative: Wang yan Pin
- Product/Service: Total solution of SARS-CoV-2 , RT-PCR test kits , Multiplex Fluorescence Staining Solution , Pathogens targeted NGS , Newborn Genetic Screening NGS , Immunochromatography POCT
- Capital (Million US $): 5,000,000RMB
- Year Established: 2011
- Total Annual Sales Volume (Million US $): US$10 Million - US$50 Million
- Export Percentage: 51% - 60%
- Total Annual Purchase Volume (Million US $): US$5 Million - US$10 Million
- No. of Production Lines: 5
- No. of R&D Staff: 71 -80 People
- No. of QC Staff: 51 -60 People
- OEM Services Provided: yes
- Factory Size (Sq.meters): 1,000-3,000 square meters
- Factory Location: 2nd Floor, No.6 Building, Xili Liuxian Culture Park, Nanshan District, Shenzhen City, Guangdong Province, China
- Contact Person: Ms. Super D~D
- Tel: +86-755-86505501
Premium Related Products
Other Products
Hot Products
Retro chinese traditional crown tiara pageant tiarasWhite Crystal Wholesale Pageant Crowns And Tiaras Hair Accessories2015 New Cheap big pageant fashion tiara crownWholesale Cheap Bridal Queen Crowns And Tiaras Jewelry TiarasReligious beads braceletwedding tiaraBeauty large crystal queen pageant crownSuper beautiful crownSuper beautiful crown Artificial diamond Fashion crown2014 big pageant crown,tall animal tiaras for sale2014 Santa Claus crown,big pageant crown,tall animal tiaras for sale2014 crowns tiaras,big pageant crown,tall animal tiaras for saleFashion holiday party crown in 2014crowns tiaras,big pageant crown,tall animal tiaras for saleFashion holiday party gold crown ringcustom pageant crowns tiara,wedding tiara and crown